In 2015, I published a paper titled “Transdermal Product Development in the Age of QbD”. In that paper, I provided an accounting of all the prescription-only, FDA-approved drug patches as of 2015. Since a number of years have passed since then, I thought it would be appropriate to audit/update those data and revisit my assertion that novel patches (APIs not previously delivered to/through the skin) are approved by the FDA at a rate of about 1 patch every other year((A concept I hinted at as early as 2011 and first presented formally in “The Role and Implications of QbD in the Topical/Transdermal Drug Development Process”, AAPS Webinar, February 2014.)).
In the process of revisiting these data, I omitted active patches (iontophoretic and self-heating patches) and I also added several non-OTC, passive patches I had overlooked that were approved by the FDA, but not launched or never widely available. Consequently, the more narrowly focused count of prescription-only, passive patches approved by the FDA by 2015 was 63 (five more than the 58 I claimed at the time) which works out to about 7 months between approvals from 1979 to 2015.
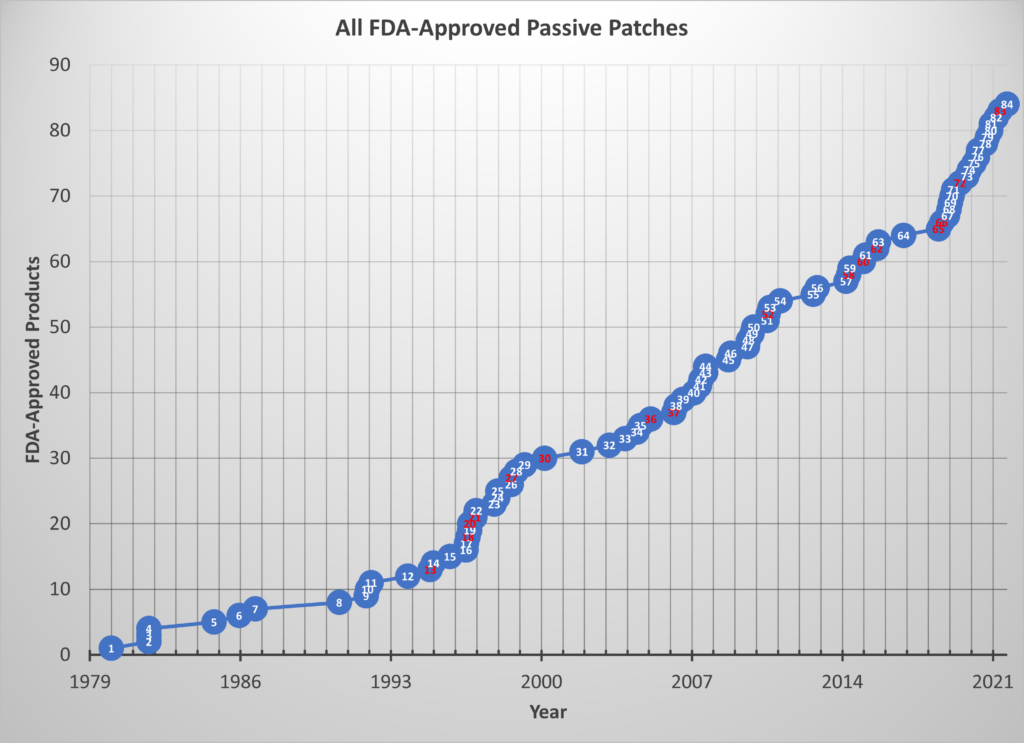
In the five-and-a-half years since the original paper was published, 21 more patches were approved bringing the total to 84 as of April 2021((I worked on 16 of these projects or about 1 in 5.)). This means the average time between approvals since 1979 is now 1 month less than it was just 5½ years ago and the average lag between approvals since 2015 is only 100 days!
The chart below shows the updated graph for all passive patch approvals. Those marked in red are the projects in which I participated.
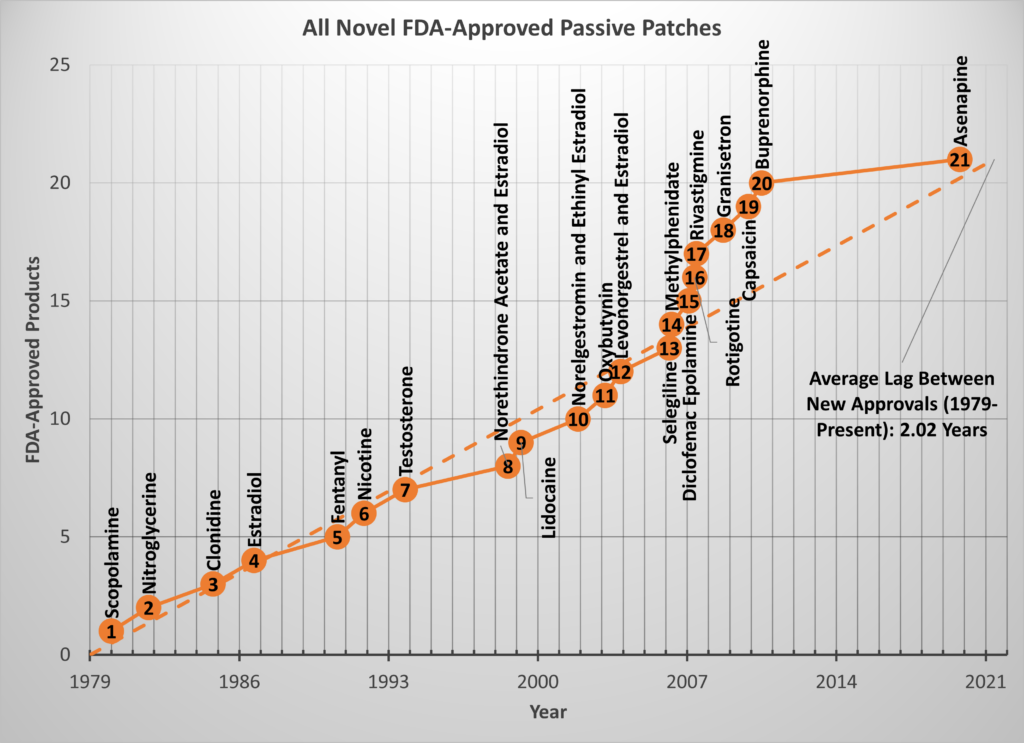
The original paper also contained a plot of the novel patch approvals (i.e., the subset of approvals for which the API was first delivered by a topical/transdermal patch). At the time of publication in 2015, I noted 24 novel patches with an approval rate of about one patch every other year. After double-checking the data, I found there were only 20 novel, passive patches approved as of 2015 which gives a rate of 1.8 years (22 months) between approvals from 1979 to 2015 (still about 1 patch every other year.) Since 2015, only one novel patch has been approved by the FDA (Secuado, asenapine transdermal system, 10/15/2019), but that is enough to continue the trend.
As of this writing, there are 21 novel, passive patches approved by the FDA over the last 42 years so the historic rate is still EXACTLY 2 years/patch.
The chart below shows all the novel API patches plotted at their respective approval dates and a broken line showing the slope of one approval every 2.02 years.
These data, of course, consider only the US market which is still (I believe) the largest market for commercial pharmaceuticals and is often the focus for product development even for developers outside the USA. Nevertheless, these data give us confidence that the field is just as active as it ever was–even as we approach half a century.
Ken Miller

